Rapid COVID tests can and will change the live events industry. However, as new tests emerge, their benefits must be weighed carefully; many offer speed at the cost of accuracy.
Rapid COVID tests can and will change the live events industry. However, as new tests emerge, their benefits must be weighed carefully; many offer speed at the cost of accuracy.
There is no doubt that effective rapid COVID tests could change the roadmap for future events, enabling participants to be tested for COVID-19 as they register for a conference. However, until an affordable and accurate test is readily available, accuracy will play runner up to speed in the rapid testing universe.
While several companies are vying for the Rosetta Stone of R&D: a rapid, at-home test, none have yet won approval. Thus far, rapid tests have proven notoriously unreliable (speed over accuracy). Just ask the Trump Administration who recently discovered that quick, easy-to- use rapid testing is no excuse for not wearing masks. The recent White House (WH) outbreak following the Rose Garden event of September 26th proved how critically important it is to wear masks regardless of testing protocols. The WH seems to have believed that tis better to catch the presence of the virus after the fact than to prevent catching it at all.
Not the best strategy at press time with 18 participants testing positive thus far…and counting.
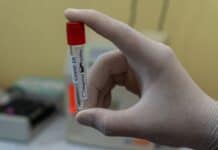
At the end of August, the FDA authorized the first emergency-use approval rapid COVID tests that don’t require any special computer equipment for fast results. Courtesy of Abbott Laboratories, the 15-minute test sells for $5, is the size of a credit card and has a competitive edge over similar tests that need to be inserted into a machine. This self-contained test utilizes the same technology used for testing for the flu, strep throat and other infections. The tests detect specific proteins — known as antigens — on the surface of the virus, and can identify those who are at the peak of infection, when virus levels are likely to be high. These tests can be rolled out in huge numbers and can spot those who are at greatest risk of spreading the disease and proponents believe antigen tests could help keep the pandemic at bay.
It’s just the latest cheaper, simpler test to hit the U.S. market and just in time as schools and businesses struggle to reopen and flu season arrives. Just recently, the FDA also greenlighted a Yale University saliva test that bypasses some of the supplies that have led to testing holdups. However, both tests have limitations and neither can be done at home.
However, a recent pilot program for a new rapid-testing technique conducted at the Austria Center Vienna offers hope to the events industry. The NADAL COVID-19 Ag Rapid Test was tested on 2,000 Vienna University students, staff members, employees of partner companies, and journalists over two days. During the September 16-17 pilot program, the test took an average of six to 10 minutes — from swabbing to receiving the test result — significantly less than the anticipated 15 minutes.
In total, five people tested positive on the first day and one tested positive on the second day of the pilot program. The person who tested positive on the second day had previously tested negative, which confirmed that an antigen test only ever provides a snapshot of an individual’s viral load at that specific time.
“No hygiene plan or testing procedure can provide full protection from COVID-19, admitted managing director Austria Center Vienna, Susanne Baumann-Söllner. “But we all firmly believe that rapid tests represent a major step in the right direction and could quickly go on to become the new standard. We want this pilot project to show the industry what new options are available and provide targeted support for organizers,” she concluded.